which of the following is a characteristic of slow twitch oxidative skeletal muscle fibers?
 Characteristics of ST, FTa and FTx muscle fibers. | Download Table
Characteristics of ST, FTa and FTx muscle fibers. | Download TableFast muscle fiber Muscle fibers are designed to feed the exhaust reactions and, as described below, they generate energy for contraction through an independent oxygen pathway that keeps the maximum development of force for about 20 years. From: Related Terms: Joseph Feher, in , 2017Lactate Chambers to Mythochondria, Oxidative Fibers or Liver The fastest muscle fibers are expected to produce lactic acid at the highest speed. The first method of deletion of lactic acid is through mytochondria in the same cells that produce it. Lactic acid is taken in mitochondria by the MCT1 and the lactic acid becomes piruvate and oxidized. This intracellular shuttle is shown in Figure 3.7.5. When the production of lactic acid exceeds the ability of lactic acid to be metabolized within the muscles of rapid traction, it is spilled into extracellular space. Some of these lactates are absorbed by oxidative fibers, which are usually smaller than large glycolytic fibers. Thus, some of the lactic acid produced and released by the muscle is metabolic in the muscle itself. This constitutes the cell transshipper, also shown in Figure 3.7.5. Figure 3.7.5. The breastfeeding transshippers. Lactate is produced in the cytoplasm by the LDH acting on the pyruvate and the NADH. Lactate can be transferred from the cytoplasmic compartment to the mitochondrial compartment by importing the lactate into the mitochondria and linking it to the synthesis of the pyruvate and the generation of NADH in the mitochondrial matrix. This is the intracellular lactate transshipper. Secondly, breastfeeding can be exported to the blood where it is absorbed by adjacent and completely oxidized muscle fibers by its mitochondria. This is the cell-to-cell shuttle. Third, lactate released in the blood when the production of lactic acid is high can be absorbed by liver cells (also called hepatocytes). Hepatocytes resynthesize glucose from the lactate and export it back to the blood where it can be taken by the exercise muscle, for example. This is the Cori cycle. The liver may take lactate that is released in the blood by active muscles. The liver metabolizes the lactate for energy or uses it to make new glucose through gluconeogenesis, and exports glucose to the blood. Muscles can then take this glucose and use it again for energy. This cycle of blood glucose to muscle lactate to blood lactate to liver lactate and back to blood glucose is called Cori cycle, shown in Figure 3.7.5. T. Lømo, in , 2009NMJs Differ in Structure and FunctionNMJs on fast and slow muscle fibers, such as the fast EDL and the slow SOL of the rat, differ both in structure and function. Precisely, the rapid nervous ends and the end of the slow nerve are arborized differently, and the rapid nervous ends contain more terminal inflammation (varicities). The quick endings release more acetylcholine at the start of the impulse trains, but since they have smaller gallbladder pools, they get tired more easily as the release runs out faster during continuous stimulation. Postsynaptically, relative to slow NMJs, fast NMJs have denser, deeper and branched infoldings. They also respond to given doses of ACh with larger post-inoptic currents. In their synaptic clefts, the rapid MJNs predominantly contain the large A12 form of AChE, while the slow MJNs contain more of the smaller A8 and A4 forms. The AChE molecules are anchored to the basal synthetic lamina by one of two types of collagen-codified collagen-based proteins of different ColQ gene transcriptions in fast and slow muscle fibers. Two different promoters have been identified for the ColQ gene, which are expressed in a differential way in fast and slow muscle fibers. The promoter present in slow fibers is activated by the transcription factor called the activated T-cell nuclear factor (NFAT) in a "low current regulatory element" (SURE), while the promoter expressed in fast fibers contains a "fast intronic regulation element" (FIRE). Assuming that the different compositions of the AChE isoforms reflect differences in function, such findings suggest that fast and slow impulse patterns transmitted by NMJs quickly and slowly raise different demands on the AChE function. Stephanie J. Valberg, in , 2008a In general, fast muscle fibers in the untrained state are biochemically appropriate to derive energy for contraction by glyco (gen)lisis anaerobic. Rapid fibers, especially type IIx, tend to have higher concentrations of glucogen and phosphate of creatine, as well as higher enzyme activities associated with glucogenolisis and glucolysis (Table 15-1). The type I fibers of slow motion, on the other hand, usually have higher concentrations of triglycerides and mioglobin and are more suitable to derive their energy through oxidative phosphorylation through the electron transport system after the oxidation of fatty acids and glucose through the Krebs cycle (Table 15-1). Type IIa fibers are intermediate in their glucolytic and oxidative capacity between type IIx and type I fibers (Adhihetty et al., 2003; Rubenstein and Kelly, 2004). Triglycerides and glucogen serve as primary substrates for muscle metabolism. In general, the rate of use of glucogens is higher with anaerobic exercise of high intensity, while low-intensity submaximal exercise results in a lower rate of use of glucogens and dependence on the oxidation of fatty acids as fuel (Kiens, 2006). In conditions of restricted energy consumption or prolonged exercise, amino acids can also serve as energy substrates within the skeletal muscle (Rennie et al., 2006). D.J. Ellerby, in , 2011 Regional variation in the properties of the myotom muscle The contactial properties of the myotom muscle vary between types of fiber, with position in the axis of the body and between stages of development. The production of strength and shortening by skeletal muscle are caused by the cross bridge cycling of myosin. This requires adenosine energy triphosphate (ATP) and the binding and release of Ca2+ troponin C, a protein component of the thick myosine filament. Muscle shortening speeds decrease in the white order pink sqm red in concert with a content of miosin-ATPase decreasing. Factors that affect the Ca2+ occupation of troponin binding sites and cross-approach and detachment rates change muscle activation rates, shortening and relaxation. Differences in activation, shortening and relaxation rates can occur independently, as they are influenced by separate muscle proteins. The red and white muscle fibers of a series of teleost species have slower activation and/or relaxation rates that move along the axis of the body from before to after. These differences have been correlated with longitudinal changes in the expression of three muscle proteins: parvalbumin, troponin T, and the miosin light chain (MLC; specifically MLC2 the regulatory light chain). Regional differences have been found in the relative amount of these proteins and/or in relative proportions of alternative protein isoforms: Ca2+ coupling to troponin C is an essential step intriguing cross bridge cyclism and muscle contraction. Parvalbumin joins Ca2+ free in myoplasm, competing with troponin C. Parvalbumin, therefore, influences muscle relaxation by reducing the concentration of free Ca2+ in myoplasm. High levels of parvalbumin should be associated with rapid muscle relaxation. The contents of Parvalbumin decreases from before to after in trucha (Oncorhynchus mykiss), sheephead (Archosargus probatocephalus), and king fish (Menticirrhus americanus) white band red muscle contract, and in cod (Gadus morhua) and under big mouth (Micropterus salmoides) muscle relax Troponements It is thought to affect the rate at which Ca2+ dissociates from troponin C, and therefore the rate at which the muscle relaxes. Relative proportions of two troponin isoforms move from pre to later in cod (G. morhua) and American perch (M. salmoides), with the previous dominant form that probably has kinetics faster than the alternative isoform. MLC2 is a thick filament component that can modulate cross bridge kinetics by controlling Ca2+ sensitivity. The amounts of the slow increase in isoform MLC2, going from prior to later on in the slow and rapid muscle of the rainbow trout (O. mykiss). It is likely that the list of variable regional proteins that influence the contactial properties of the myotom muscle will grow as more data is available. There is less data available for elasmobranchs, but similar regional differences have not yet been detected in contactial properties. In addition to regional mechanical differences, there is also variation to the extent that the muscle fibers of fish depend on aerobic or anaerobic metabolic pathways to supply energy for contraction. The volume fraction of red fibers occupied by mitochondria is generally greater than 25% compared to less than 10% in the white muscle. As a result, the activities of mytochondrial enzymes associated with aerobic metabolism, such as cytochrome oxidase and citrus syntase, are higher in red than in the white muscle. The pink muscle shows an intermediate level of activity for these enzymes. The white muscle is mainly based on anaerobic glycolysis for its energy supply, as indicated in higher levels of glycolytic enzymes, such as phosphoructokinase, which is found in the red or pink muscle. Zsolt Radák, in , 20182.2 Types of muscle fibers Slow and fast muscle fibers have already been briefly addressed; here we discuss them in detail. The muscle is composed of different muscle fibers, which differ in appearance and other characteristics. For example, comparing isolated muscle of a wild and domestic rabbit, the wild is more reddish in color. Also when comparing the chicken breast with the thigh, the latter is more red than the chest. Thus, the muscle that is exposed to constant activity (such as the muscle of a wild rabbit, and the musles of the chicken) is reddish, and is composed of slow muscle fibers, while the muscles that are not exposed to constant effort (muscle of a domesticated rabbit, and the chicken breast) are lighter in color and are composed of fast-stretched muscle fibers. The occurrence of muscle fibers depends on effort, inervation and the type of inervation. Within a muscle different types of muscle fibers may appear — for example, closer to the bones, muscles are more reddish than near the surface. In general terms, extenders contain faster muscle fibers than flexors. There are muscles in the human body that are mainly composed of slow muscle fibers or rapid traction. Muscle fibers are inervaded by alpha motor neurons. The motor neuron and all the muscle fibers it connects to is a drive of motor. The number of muscle fibers inervaded by a motor neuron may differ; for example, in extraocular muscles, 10 muscle fibers are inervaded by a motor neuron, while the muscles of the thigh can have 1000 fibers in each unit. The axes of spinal cord motor neurons invade peripheral muscles, and may have a length of more than 1 m (Fig. 2.9). Engine units. There are three different motor units in the human body. The type I motor is highly fatigue resistant, has a lower activation threshold, contains fewer muscle fibers, and has low strength generation during contraction. The type II motor unit is also fatigue resistant, has a higher activation threshold, and the force produced is higher compared to type I. The type IIb motor unit is fatigueable, has a high activation threshold, internalizes the most muscular fibers, and generates the greatest strength during contraction. Engine units differ according to the size and threshold of activation. Large motor units have higher activation thresholds and contain faster muscle agitation fibers, while small motor units have lower activation thresholds and contain slow reddish muscle fibers. Differences in physiological, biochemical, histochemical and genetic characteristics of motor units also provide a useful basis for distinguishing between them (Table 2.2). Table 2.2. Medium strengthsHigh strengths Another classification gives another type of fibers, fibers IIi, with characteristics between type IIa and IIb. Slow red wire fibers contain high amounts of iron, which come from the largest number of mitochondria and mioglobin content. Red fibers have greater oxidative capacity; they are able to consume large amounts of oxygen and reduce it in mitochondria. Oxygen is always linked to iron-containing molecules; this high iron content also contributes to its red color. Table 2.2 shows the differences between types of muscle fibers. The differences in the activation threshold determine the activation order of the different types of fiber in contraction. Slow muscle fibers with their excellent oxygen consumption rate and high mitochondria content and oxidative enzyme activity are the most efficient fibers. They are able to generate strength at the contraction point due to their low activation threshold. Most of the fibers of the anti-gravity muscles are slow fibers and these fibers are involved during low-intensity movements and walking. One of the main laws in nature is profitability, which in this case means the commitment of the most profitable muscle fibers first. Fast muscle fibers with their higher activation thresholds and huge force generation are usable during flight and survival; however, these fibers consume a lot of energy and produce a lot of dactic acid (unknown later). They can only be activated by high intensity stimuli due to the upper threshold. To use an analogy, slow traction fibers are like cars from the economic city, while fast muscle fibers are like high-powered racing cars. Stefano Schiaffino, Carlo Reggiani, in , 2012 Mechanical properties during contractions and rest: sarcomeric motors and Cytoskeleton The low daily amount of activity of fast muscle fibers is closely related to their specialization with contractile, as such fibers are able to develop high tension and high mechanical power and are therefore used for fast and strong movements. However, slow fibers are used in tasks that require less energy and tension, but specialize in minimizing energy costs and avoiding fatigue. The molecular basis of specialized contractile performance can be found in the specific isoforms of sarcomeric proteins expressed by fast and slow fibers (Figure 60.4). In fact, the conversion of ATP chemical energy into mechanical energy (work) and heat occurs at different rates mainly depending on the specific myosine isoforms. The fast fibers, with their 2A, 2X and 2B subtypes, develop more tension than the slow fibers during the maximum contraction (50.51) and, if it is allowed to shorten, reach a higher speed of shortening against any given load, thus producing different speed of force (50) (Figure 60.5A). In addition, the mechanical power, which is the rate of labor generation and is the most relevant parameter for the movement of members or the body, reaches different values in relation to the isoform of myosin expressed by the fibers (Figure 60.5B). Shortening speed and maximum power values gradually increase from slow to fast 2A, fast 2X and fast 2B. The ATP hydrolysis rate also differs between slow and fast fibers. The cost of developing the voltage in ATP is much lower in slowness than in fast fibers (53.54), while the ATP hydrolysis rate during shortening is approximately in proportion to the output of energy and this implies a similar conversion efficiency of chemical-mechanical energy regardless of the isoform expression of myosine (55). In general, the mechanical and energetic parameters of the contractual response make slow fibers more suitable for low-intensity and long-lasting activity and fast fibers for short and strong contractile performance. Figure 60.4. Main contradictory and cytosceletal proteins of sarcomere showing differential distribution between rapid and slow muscles. Prevalent forms in slow muscles are indicated at the top of the figure, quick isoforms at the bottom. Slow muscles are also characterized by a longer nebulin and isoforms of titin compared to rapid muscles. FHL1, four and a half domains LIM 1; MLP, muscle LIM protein; TM, troomyosin; Tn, troponin; MyHC, heavy myosin chain; MyLC, light myosin chain; MyBP-C, myosin binding protein C.Figure 60.5. Diversity of functional parameters between types of muscle fiber.(A) strength curves of individual rat fibers, maximally calcium activated at 12 °C, classified according to its content of isoform of myosine (redged from data reported in Bottinelli et al. (50), table 3, according to equation V=Vo.b.(1−P/Po)/(P/Po+a). (B) Power strength curves of the same four types of muscle fibers of rat (following data as in panel A, equation W′=P.V.Po). (C) Average cyto-olylic calcium transients after a single electrical stimulus detected with MagFluo-4AM in the four types of skeletal muscle fiber of the mouse, identified on the basis of its isoform content of myosin. The range of peaks has been equaled in all transients to better show the diversity in the decay kinetic. (D) Lactate dehydrogenase (LDH) and hydroxyacyl CoA dehydrogenase (HADH) activities in individual fibers of plantaris rat assigned to their types on the basis of ATPase staining after alkali and acid preincubation. Mioborilos, where the interaction of myosin and actin produces strength and movement, are part of the cytoskeleton, that is, the cellular scaffold responsible not only for determining the form and size (length) of the muscle fibers, but also for transmitting the strength and movement to the extracellular fibrous skeleton. The diversity between fibers is also detectable in cytoeskeleton, in particular in the aggregates of transverse proteins that form the Z-disks, M bands, and in longitudinally oriented sarcomeric giant proteins (titine and nebulin) (Figure 60.4). Slow fibers have thicker Z-discs and M-band, a feature that is probably related to the ability to withstand active force (56). Titin, the main determinant of the rest tension, is present in slow fibers with longer and extendable isoforms, thus allowing passive elongation with less mechanical resistance compared to fast fibers (different curve of length of rest tension and viscoelasticity) (57). Slow fibers are also characterized by a longer nebulae and actin filaments, which might imply that the active voltage length curve and optimal length are shifted towards a longer sarcomere length (57.58). It should be recalled that, although the rapid fibers develop greater tension during the isometric contractions, the difference disappears in the eccentric contractions where the highest forces are generated (59). In such conditions, the ability to withstand tension without damage is greater in slowness than in fast fibers (60). Margaret Buckingham, Alicia Mayeuf, in , 2012 Quick versus fast slow muscle regulation In the adult muscle, Six1 and Eya1 accumulate in fast muscle fibers, and the ectopian overexpression of these factors in the slow-type sunny muscle results in a conversion of the slow oxidative phenotype to a fast glycolytic phenotype. The upper activity of the Six1/Eya1in rapid-type fibers is observed immediately after delivery, suggesting that it may have a pre-contesting role in triggering the fast-type program, before slow/fast neuron activity. A transcription analysis in the embryo shows that many genes that shrink rapid muscle isoforms are deregulated in the remaining miotome of 61/4 double mutants. Six1/4 binding to regulatory regions of fast muscle genes is compatible with direct regulation for these factors in embryo as well as adult (3). In the mouse, fast and slow isoforms are co-expressed in embryonic muscle cells. This contrasts with the situation in the zebra fish, for example, where there is an early segregation of fast and slow muscle progenitors. In this case, Hedgehog's signaling induces the transcriptional repressor, Prdm1/Blimp1, in adaxial cells, avoiding the activation of fast muscle genes and the gene for Sox6, a slow-type genetic transcription repressor, thus conferring a slow phenotype on these cells. Prdm1 is expressed in the miotome of the mouse, but mutants do not have a fast phenotype, reflecting differences in the regulation of slow muscle genes; in myotom cells. Sox6 null embryos only show the regulation of the slow expression of the muscle gene in fetal stages (4). Zsolt Radák, in , 20184.3 Drives and Power Generation There is a significant difference between slow and fast muscle fibers, as discussed in chapter 2, on their activation threshold and power generation. A question arises about why motor units should be distinguished from the entire muscle. Unlike the heart muscle where all heart cells work as a syncitium and there are no motor units, in the skeletal muscle, motor units (muscular fibers inervaded by a single neuron) are recruited and activated. The generation of skeletal muscle strength is several hundred times greater than that of the heart muscle, which requires a complex inervation. Recruitment and synchronization of motor units can be measured by electromyography (EMG); however, motor units types can only be determined indirectly on the basis of physiological characteristics such as fatigue and force range. As mentioned above, muscle characteristics depend on inervation, which can be modified to a limited degree. The recruitment of motor units begins with the small ones, which generate the least strength, and is followed by motor units that are larger in size and generate a significant force (Zajac and Faden, 1985). This indicates that the recruitment of motor units aligns with the power necessary for a movement; for example, there is no recruitment of large engine units if a 5% force is required for a movement ensuring profitability. When a huge power is needed, in addition to fatigue-resistant engine units, other fatigued engine units are recruited with higher activation thresholds. Thus, the generation of force increases with the recruitment of motor units in a non-linear way, since the generation of force by the different units is not equal. The correlation is exponential as the most powerful motor units are recruited for the last time (Fig. 4.1). Fig. 4.1. Correlation between load and recruitment of motor units. Low-performance motor units (type I) are first recruited by their low threshold. If a greater force is needed, more and more intermediate engine units (type IIa) are activated, then fast motor units (type IIb) with high activation thresholds are finally registered. Slow fibers generate the least strength, while fast fibers generate the greatest strength. The strength generated by different muscle fibers differs significantly even if a muscle, for example, the soleus, mainly contains slow fibers; the generation of strength falls between 31 and 1600 mN (McDonagh et al., 1980). This difference can be extreme in the case of a muscle composed mainly of fast agitation fibers, for example, the previous tibialis. It should be noted that the generation of force can differ between the same types of motor fibers, which can be monitored by EMG, where the fiber types are reflected by activation frequency. The activation order mentioned can be seen in slow movements, while in rapid movements there has been a recruitment dependent on tasks. In the latter case, for example, the movements learned, the rapid motor units can be recruited previously regardless of the normal activation order (Herrmann and Flanders, 1998). This observation highlights the potential utility of smooth automatic movements in explosive force training. This will be further discussed through the formation of explosive force. N.H. Casey, E.C. Webb, in , 2003p The HGoat muscle contains aerobic (red) and anaerobic (white) muscle fibers and suffers the same postmortem biochemical changes as meat and mutton. The decrease of the goat muscle pH follows a typical pattern of red housings to stabilize around pH 5.4. Variations occur due to differences between muscles, sexes and premortem stress. The stress of exhaustive premortem produces dark, firm and dry meat with a maximum pH (pH ю 6.0). Postmortem biochemical changes are associated with the loss of water binding capacity, since pH reaches the isoelectric point of muscle proteins, the beginning of rigor mortis, and the release and activation of protein enzymes, especially caepsins, responsible for the ripening of the meat. Recommended Publications: We use cookies to help provide and improve our personalized service and content and ads. By continuing to accept . Copyright © 2021 Elsevier B.V. or its licensors or collaborators. Direct Science ® is a registered trademark of Elsevier B.V.ScienceDirect ® is a registered trademark of Elsevier B.V.

32. FAST AND SLOW TWITCH SKELETAL MUSCLE FIBERS Flashcards | Quizlet

Types of Skeletal Muscle Fibers

Skeletal muscle fibre types and their characteristics Flashcards | Quizlet

What's the difference between fast and slow twitch muscle fibers? - FitnessGenes®

Solved: 15.) Circle All Of The Characteristics Listed Belo... | Chegg.com

Types of Skeletal Muscle Fibers

Types of Skeletal Muscle Fibers | Lifetime Fitness and Wellness
/fast-slow-twitch-muscle-596cebfc685fbe001130a49e.png)
Fast and Slow Twitch Muscle Fiber Types
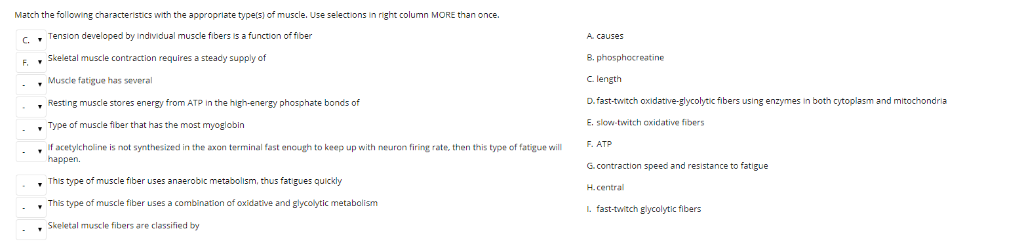
Solved: Match The Following Characteristics With The Appro... | Chegg.com

Quiz & Worksheet - Skeletal Muscle Fibers | Study.com
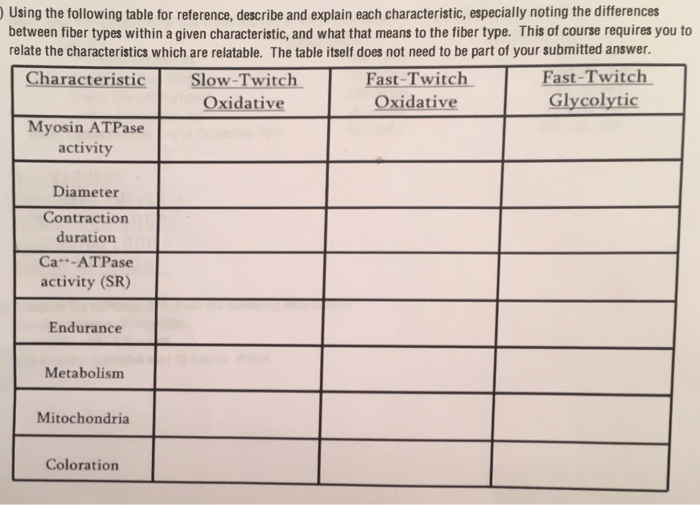
Solved: Using The Following Table For Reference, Describe ... | Chegg.com

Solved: 15.) Circle All Of The Characteristics Listed Belo... | Chegg.com
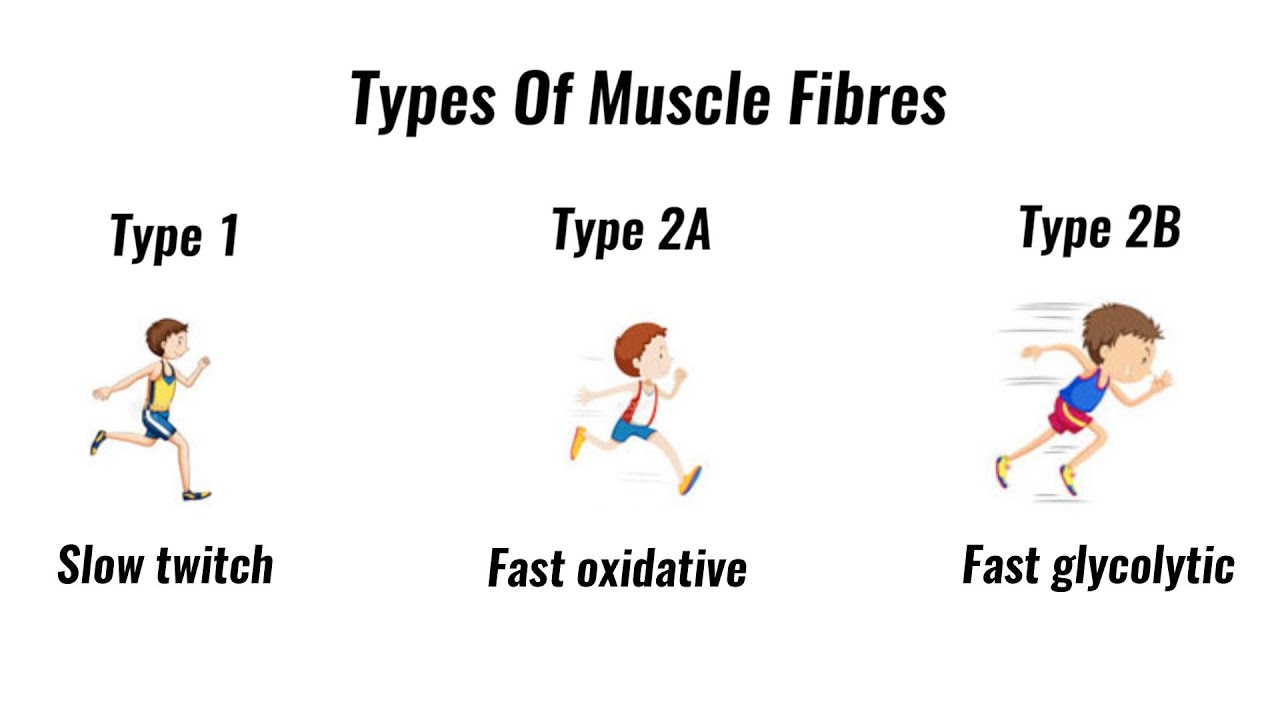
Types of muscle fibres - fast twitch, slow twitch (GCSE PE) - YouTube

Fast Muscle Fiber - an overview | ScienceDirect Topics
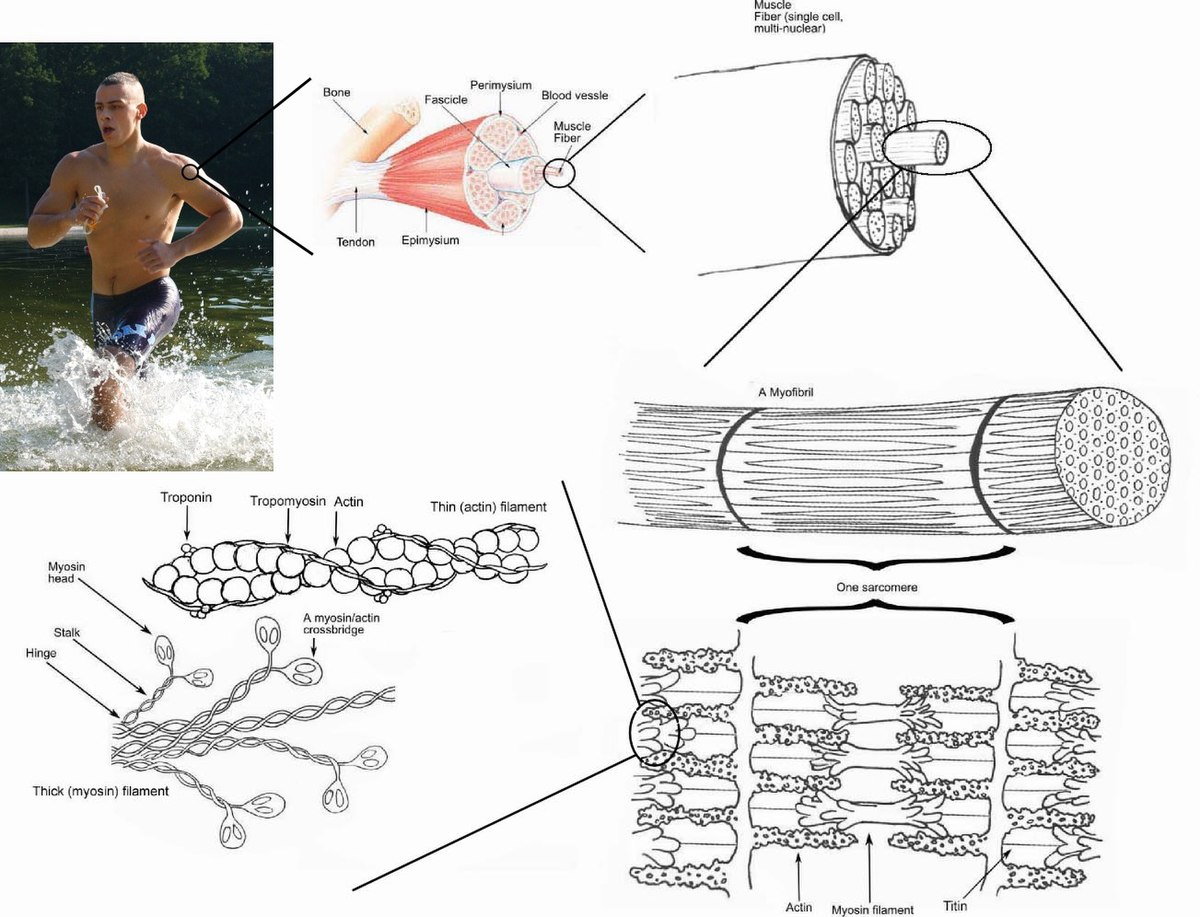
Skeletal muscle - Wikipedia

Muscle Fibre Types - Fast Twitch, Slow Twitch - TeachPE.com

Type 1 and type 2 muscle fibers (video) | Khan Academy

Type 1 and type 2 muscle fibers (video) | Khan Academy

Fnip1 regulates skeletal muscle fiber type specification, fatigue resistance, and susceptibility to muscular dystrophy. - Abstract - Europe PMC
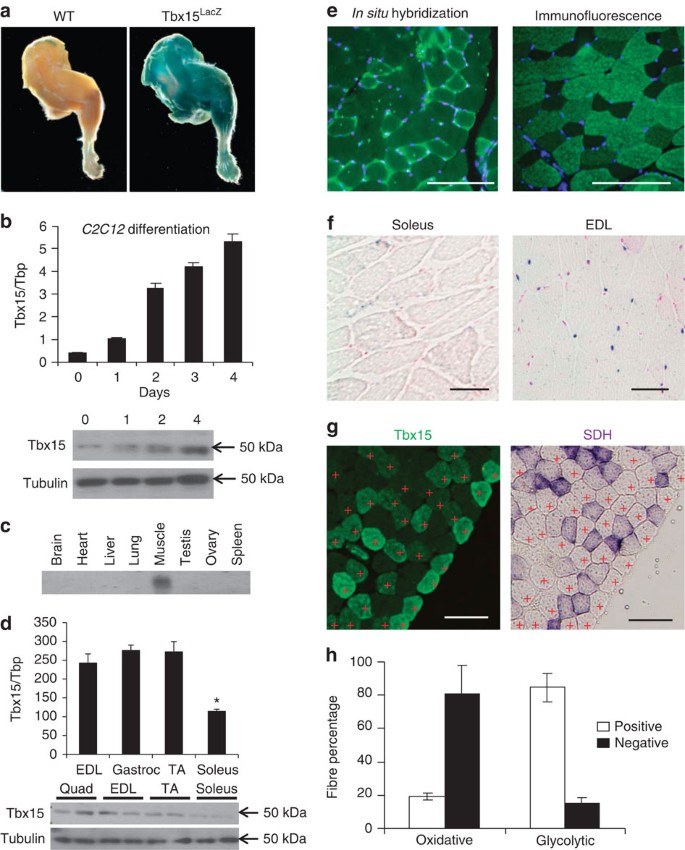
Tbx15 controls skeletal muscle fibre-type determination and muscle metabolism | Nature Communications
![Skeletal muscle Nur77 expression enhances oxidative metabolism and substrate utilization[S] - Journal of Lipid Research Skeletal muscle Nur77 expression enhances oxidative metabolism and substrate utilization[S] - Journal of Lipid Research](https://els-jbs-prod-cdn.jbs.elsevierhealth.com/cms/asset/da07307f-06c3-4bc3-975d-a5ad91cf790a/gr1.jpg)
Skeletal muscle Nur77 expression enhances oxidative metabolism and substrate utilization[S] - Journal of Lipid Research

Skeletal muscle: A review of molecular structure and function, in health and disease - Mukund - 2020 - WIREs Systems Biology and Medicine - Wiley Online Library

Fast Muscle Fiber - an overview | ScienceDirect Topics
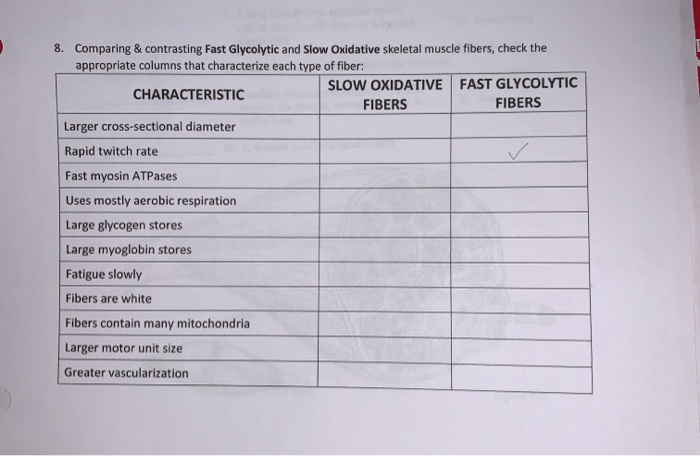
Solved: 8. Comparing & Contrasting Fast Glycolytic And Slo... | Chegg.com

AK Lectures - Slow Oxidative, Fast Oxidative and Fast Glycolytic Muscles

Skeletal Muscle Fibers: Types and Functions - Video & Lesson Transcript | Study.com

Stimulation of Slow Skeletal Muscle Fiber Gene Expression by Calcineurin in Vivo * - Journal of Biological Chemistry
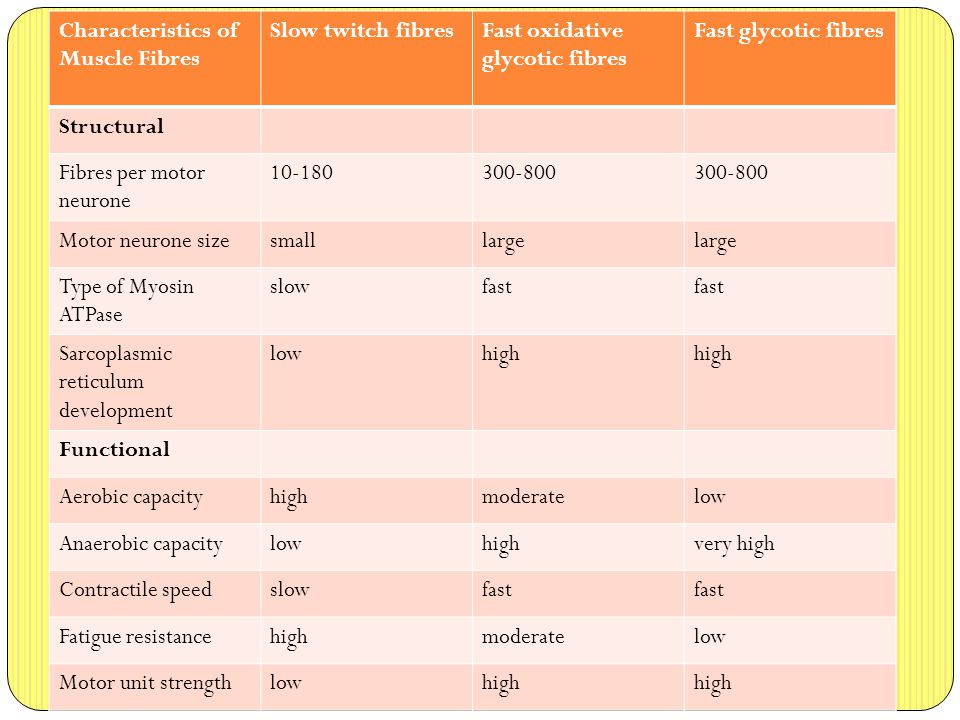
Types of Muscle Fibre Learning Objectives: - ppt video online download
Slow" skeletal muscles across vertebrate species | Cell & Bioscience | Full Text
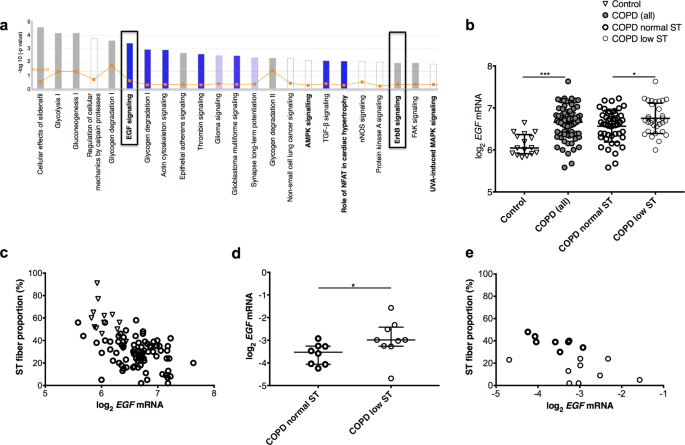
EGF receptor (EGFR) inhibition promotes a slow-twitch oxidative, over a fast-twitch, muscle phenotype | Scientific Reports
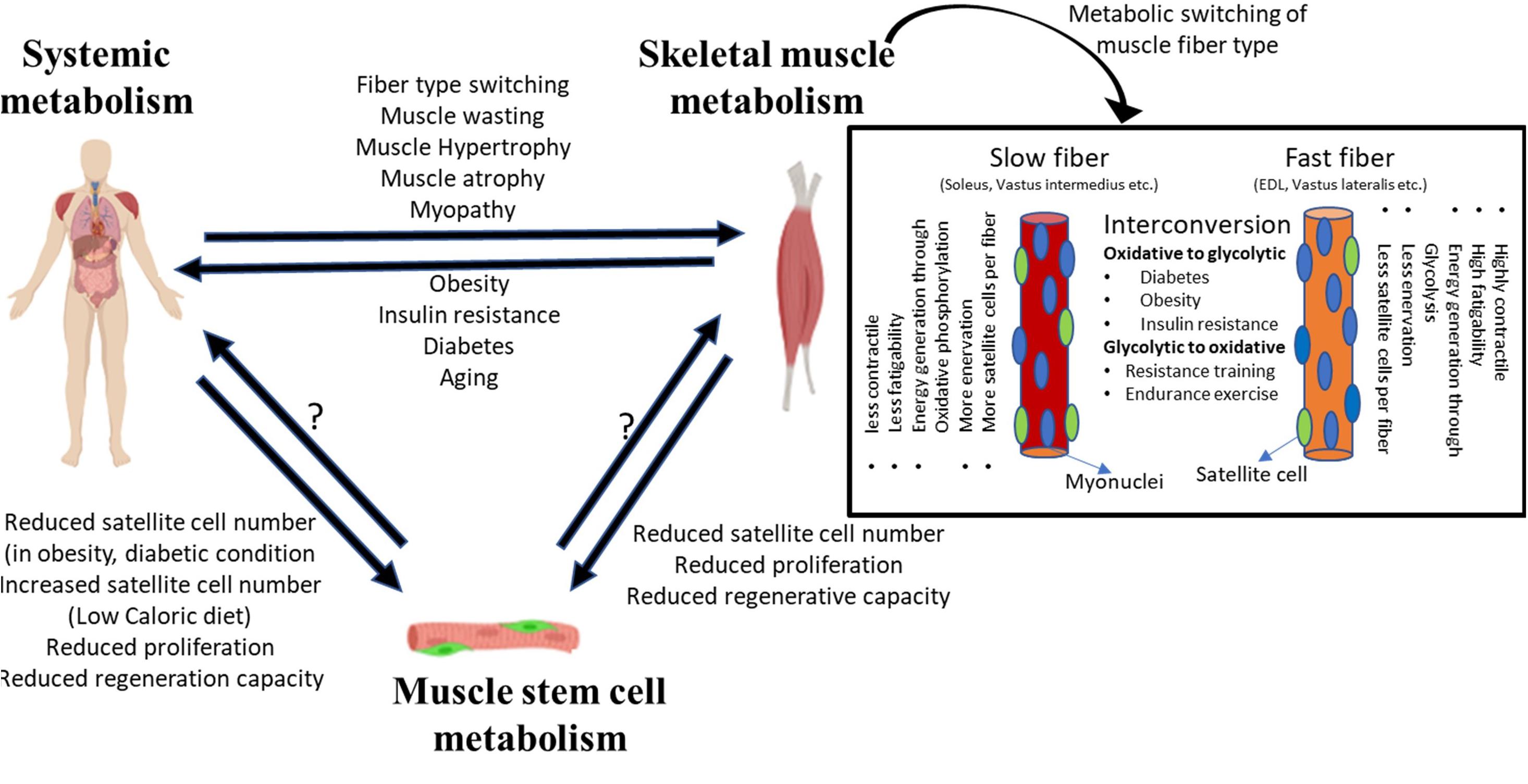
Frontiers | Adult Muscle Stem Cells: Exploring the Links Between Systemic and Cellular Metabolism | Cell and Developmental Biology

Antioxidants | Free Full-Text | Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle | HTML
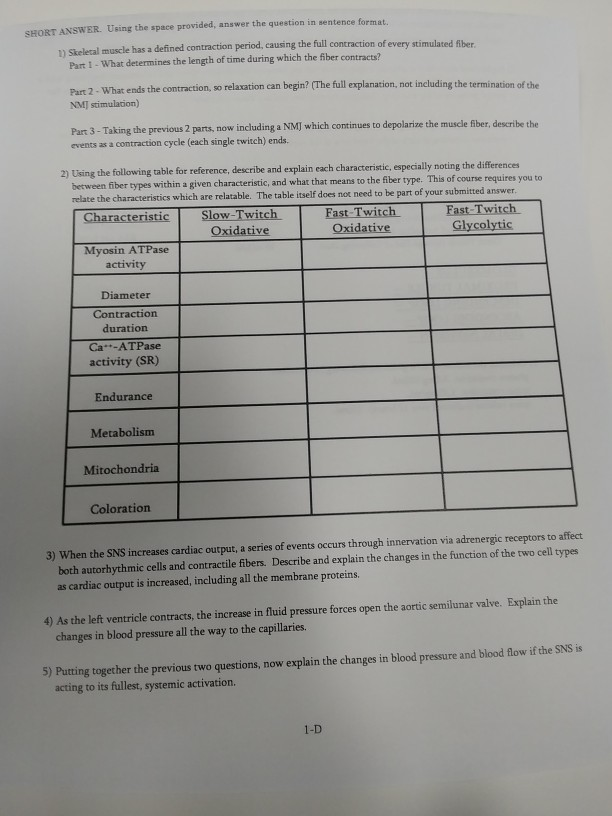
Solved: SHORT ANSwER. Using The Space Provided, Answer The... | Chegg.com

PDF) Skeletal muscle fiber characteristics of world class shot-putters
Slow" skeletal muscles across vertebrate species | Cell & Bioscience | Full Text

Fnip1 regulates skeletal muscle fiber type specification, fatigue resistance, and susceptibility to muscular dystrophy. - Abstract - Europe PMC
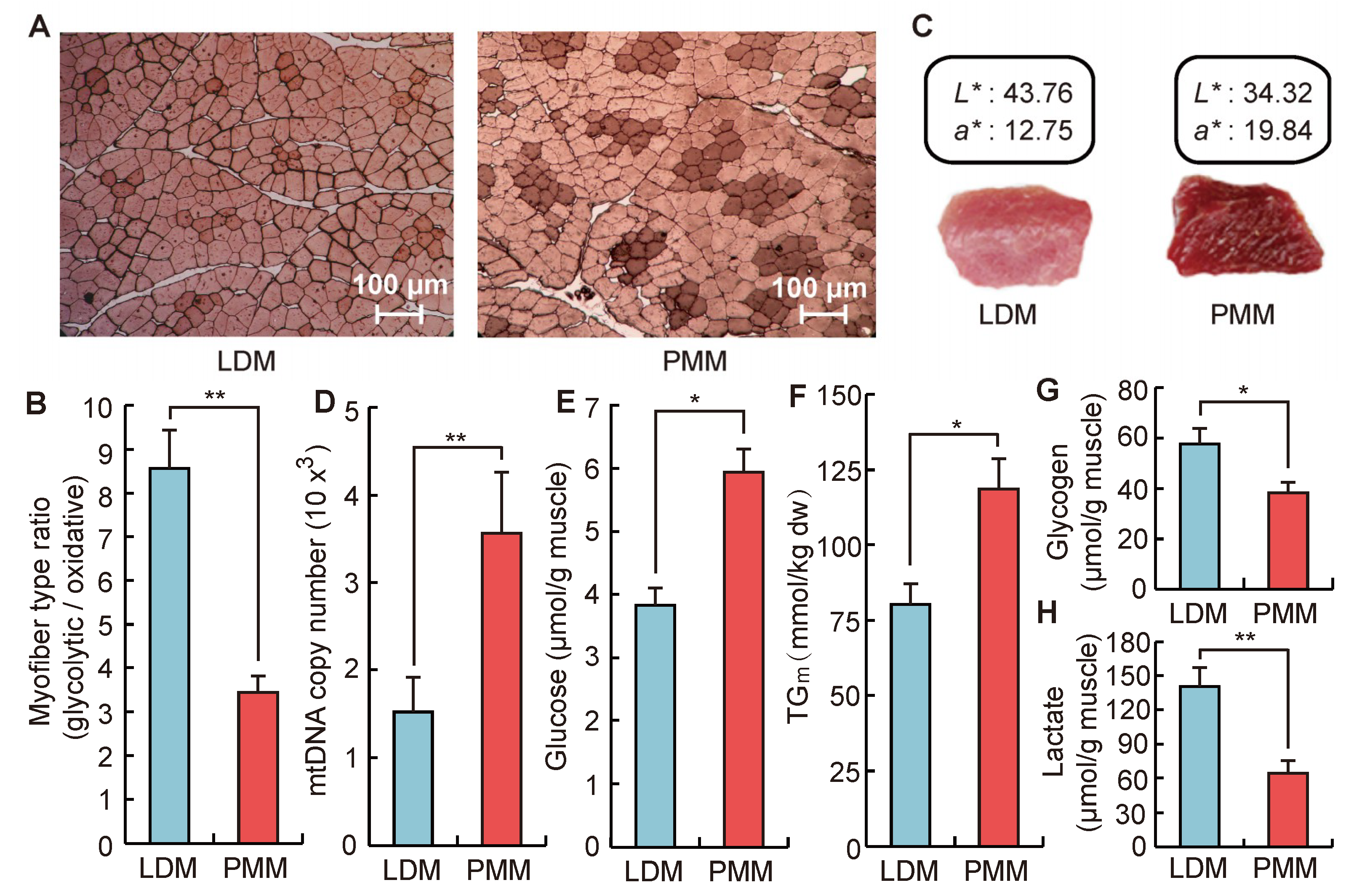
IJMS | Free Full-Text | Comprehensive Analysis of lncRNAs and circRNAs Reveals the Metabolic Specialization in Oxidative and Glycolytic Skeletal Muscles | HTML

Stimulation of Slow Skeletal Muscle Fiber Gene Expression by Calcineurin in Vivo * - Journal of Biological Chemistry

The distinct transcriptomes of fast-twitch and slow-twitch muscles in Mongolian horses - ScienceDirect

Fast-Twitch Vs. Slow-Twitch Muscle Fiber Types + Training Tips
Posting Komentar untuk "which of the following is a characteristic of slow twitch oxidative skeletal muscle fibers?"