lunesta half life
 Prescription Sleep Aids for the Treatment of Insomnia
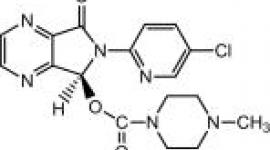
Prescription Sleep Aids for the Treatment of InsomniaSzopicloneSzopicloneClinical dataLunesta, Eszop, others/ By mouth () Legal status data52-59% and methylation (and -mediate)6 hours CID (EPA) Chemical and physical dataC17H17ClN6O3388.81 g·mol−13D model () .mw-parser-output .nobold{font-weight:normal} Eszopiclone, sold under the name of the Mondayta brand among others, is a medication used in the treatment of . Evidence bears a mild to moderate benefit up to six months. It's taken orally. SzopicloneLunestaCommon side effects include headache, nausea and dizziness. Serious side effects may include , abuse, , and . Greater attention is recommended in those with liver problems and older people. The rapid decrease in the dose can lead to withdrawal. Eszopiclone is classified as a and as a . It is the S- of. It works by interacting with it. Approved for medical use in the United States in 2004, szopiclone is available as . In 2017, it was the 214th most commonly prescribed medicine in the United States, with more than two million recipes. The szopiclone is not sold in the , as in 2009 the ruled that it was too similar to zopiclone to be considered a new patentable product. Medical Uses[]A 2018 Cochrane's review found that it produced a moderate improvement in the start of sleep and maintenance. The authors suggest that when preferred non-pharmacologic treatment strategies have been exhausted, szopicone provides efficient treatment for insomnia. In 2014, the USFDA requested that the initial dose be dropped from 2 to 1 milligrams after it was observed in a study that even 8 hours after taking the medication at night, some people were unable to cope with their next-day activities such as driving and other activities that require full alertness. Szopiclone is slightly effective in the treatment of insomnia where the difficulty of sleeping is the main complaint. Kirsch et al. found that the benefit on placebo is of questionable clinical significance. Although the effect of the drug and placebo response were rather small and of questionable clinical significance, both together produce a reasonably large clinical response. It is not recommended for chronic use in the elderly. Older[ Essential phytopathotic drugs, including szopicno, are more commonly prescribed to the elderly than to the younger patients, despite the benefits of the medication usually does not impress. Care should be taken in the choice of an appropriate hypnotic medication and if drug therapy is initiated, it should be started with the lowest possible dose to minimize side effects. In 2015, safety information on szopicone and similar drugs was reviewed and it was concluded that "agonist hypernotic non-benzodiazepine, benzodiazepine receptor (szopiclone, , ) should be avoided without taking into account the duration of use due to its association with balanced damage with its minimal efficacy in the treatment of insomnia." The review made this determination both by the relatively large dangers for older people of zolpidem and other "Z-drugs" along with the fact that the drugs have "minimum performance in the treatment of insomnia". This was a change in the 2012 AGS recommendation, which suggested limiting use to 90 days or less. The review stated: "the 90-day cavernat of use [was] removed from the hypnotic agonist of the benzodiazepine receptors, which led to an unequivocal declaration of 'avoide' (without caveat) due to the increase in the evidence of damage in this area since the 2012 update." An extensive review of medical literature on insomnia management and the elderly found that there is considerable evidence of the efficacy and durability of non-drug treatments for insomnia in adults of all ages and that these interventions are underutilized. Compared to benzodiazepines, sedative hypnotics, including szopiclone, seemed to offer few, if any, significant clinical advantages in efficacy or tolerance in older people. It was found that newer agents with new action mechanisms and improved safety profiles, such as , have the promise to manage chronic insomnia in older people. The long-term use of sedative hypnotic for insomnia lacks a basis of evidence and has traditionally been discouraged for reasons that include concerns about such potential adverse effects such as cognitive impairment (), diurnal sedation, motor incoordination and increased risk of falls and falls. Moreover, the effectiveness and security of the long-term use of these agents remain to be determined. It was concluded that more research is needed to assess the long-term effects of treatment and the management strategy most appropriate for older persons with chronic insomnia. A meta-analysis 2009 found a higher rate of . Adverse effects[ ] Sleep pills, including szopiclone, have been associated with increased risk of death. szopiclone is a contraindication to its use. Some side effects are more common than others. Recommendations on the use of szopiclone may be altered by other health conditions. These conditions or circumstances may occur in people who have decreased and other conditions. The presence of liver deficiency, breastfeeding and activities that require mental alert (e.g., driving) can be considered when the frequency and dose is determined. A meta-analysis 2009 found a 44% higher rate of mild, like or , in people taking szopiclone or other hypnotic drugs compared to those taking a placebo. Unit[] In the United States szopiclone is a controlled substance program IV under . Using szopiclone can lead to physical and psychological dependence. The risk of abuse and dependence increases with the dose and duration of the use and concomitant use of other psychoactive drugs. The risk is also higher in patients with a history of psychiatric or other disorders or a history. Tolerance can be developed after repeated use of benzodiazepines and medications similar to benzodiazepines for a few weeks. A study funded and conducted by the patient found no signs of tolerance or dependence in a group of patients followed for up to six months. Abuse[] A study of the potential for szopiclone abuse found that in people with a known history of benzodiacepine abuse, szopicone in doses of 6 and 12 mg produced similar effects to those of 20 mg. The study found that in these doses that are two or more times greater than the recommended maximum doses, an increase was observed related to the dose of amnesia, sedation, drowsiness and hallucinations for both szopicone (Lunesta) and diazepam (). Overdose[]According to U.S. prescription information, szopiclone overdose has been reported up to 90 times the recommended dose in which the patient recovered completely. According to the May 2014 issue of the official information of the United States on the prescription, only fatal victims have been reported in cases where szopicone was combined with other drugs or alcohol. He reported that between 2005 and 2006 there were 525 total szopiclone overdose recorded in the state, most of which were intentional. If consumed in the last hour, szopiclone overdose can be treated with management or pathway. Interactions[]There is a greater risk of szopiclone being taken along with other CNS depressive agents, including , sedative hypnotics (such as or benzodiazepines), , and some antidepressants. It also increases the risk of depression of the central nervous system with other medicines that inhibit the metabolic activities of the enzyme system of the . Medicines that inhibit this enzyme system include , , , and . Alcohol also has an additive effect when used simultaneously with szopiclone. Eszopiclone is more effective if not taken after a heavy food with high fat content. Pharmacology[]Eszopiclone acts on a binding site located in a . The szopicone is absorbed quickly after oral administration, with serum levels between .45 and 1.3 hours. The half life of szopiclone removal is approximately 6 hours and is widely metabolized by oxidation and demethylation. About 52% to 59% of a dose is weakly linked to plasma protein. Cytochrome P450 (CYP) isozymes and are involved in szopiclone biotransformation; therefore, drugs that induce or inhibit these CYP isozimas can affect szopiclone metabolism. Less than 10% of the oral dose is excreted in the urine as cytmic zopiclone. In terms of bonding benzodiazepine receptors and relevant power, 3 mg szopiclone is equivalent to 10 mg of . History ]In a 2009 polemic article in the , "Lost in Transmission — FDA Drug Information That Never Reaches Clinicians", it was reported that the largest of three trials in Mondayta found that compared to placebo Mondayta "was superior to placebo" while only shortened the initial time he slept in 15 minutes on average. "The cyclic who are interested in drug effectiveness cannot find effective label information: it only states that Mondayta is superior to placebo. The medical review of the FDA provides data of efficacy, although not up to page 306 of the 403-page document. In the longer phase 3 trial, patients in the Mondayta group reported that they were asleep on average 15 minutes faster and that they slept on average 37 minutes more than those in the placebo group. On average, however, the patients of Mondayta still met criteria for insomnia and did not report a clinically significant improvement in the alert or operation of the next day. " Availability in Europe[]On September 11, 2007, Sepracor signed a marketing agreement with the British pharmaceutical company for the sales rights of szopiclone (under the name Lunivia instead of Mondayta) in . Sepracor was expected to receive approximately 155 million dollars if the agreement passed here. In 2008 Sepracor submitted a request to (the European Union equivalent to the United States) for authorization to market the medication in the EU, and initially received a favorable response. However, Sepracor withdrew its authorization request in 2009 after the EMA stated that it would not grant the status of szopiclone "new active substance", as it was essentially pharmacological and therapeutically too similar to being considered a new patentable product. Since zopiclone has expired, this ruling would have allowed rival companies to produce also legally cheaper versions of szopiclone for the European market. As of November 2012, Sepracor has not submitted its request for authorization and szopicone is not available in Europe. The agreement with GSK fell, and GSK launched a $3.3 billion agreement to the Actelion sleeping tablet market, which entered phase 3 medical tests before development was abandoned due to side effects. [] ##################### ##################################################################################################################################################################################################################################### Other Other Other Other Other ( ) Several constituents / Other/non-assorted See also: • See also: Navigation menu Personal tools Named spaces Variants Views More Search Navigation Contributed Tools Printing/exporting Languages

Lunesta (Eszopiclone): Uses, Dosage, Side Effects, Interactions, Warning

Eszopiclone - Wikipedia
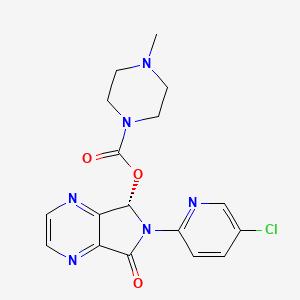
Eszopiclone | C17H17ClN6O3 - PubChem
Lunesta vs Ambien: Main Differences and Similarities

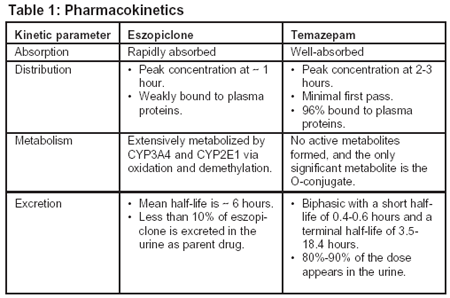
Pharmacokinetic profiles of Z-drugs | Download Table

Lunesta Tablets (Sepracor), Drug Reference Encyclopedia

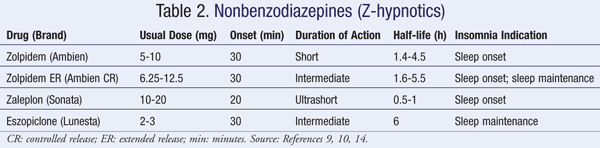
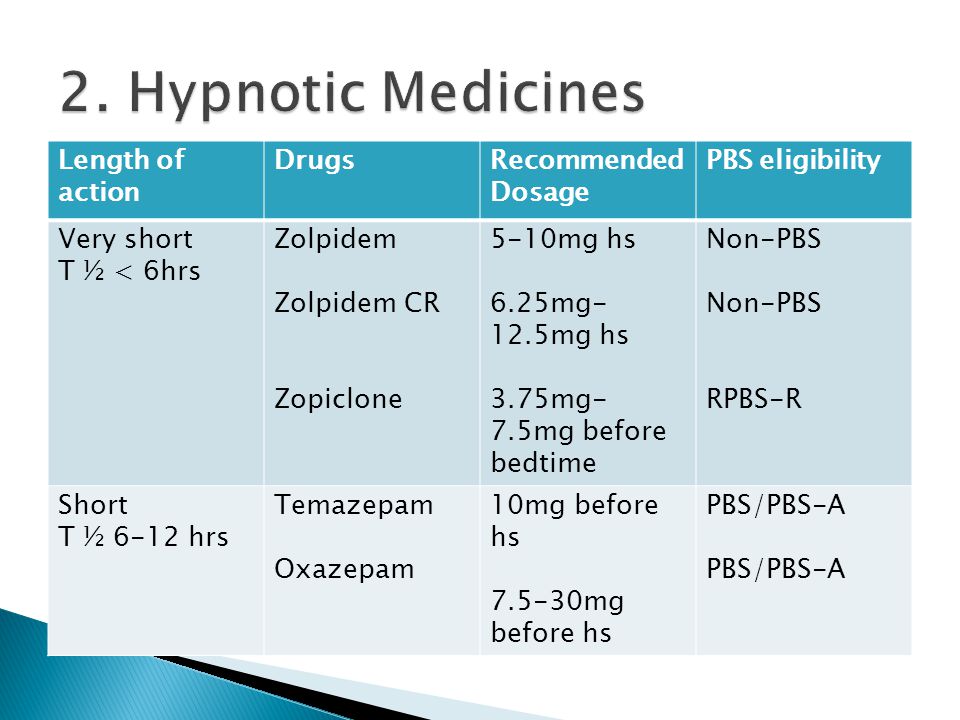
Sedative & Hypnotic Drugs Part II

How Long Does Lunesta (Eszopiclone) Stay In Your System?

Lunesta - FDA prescribing information, side effects and uses
Assisting Seniors With Insomnia: A Comprehensive Approach
/GettyImages-583640768-55d14205ab6d4cb8b29cfeec5fee2899.jpg)
Stopping Sleeping Pills and Rebound Insomnia
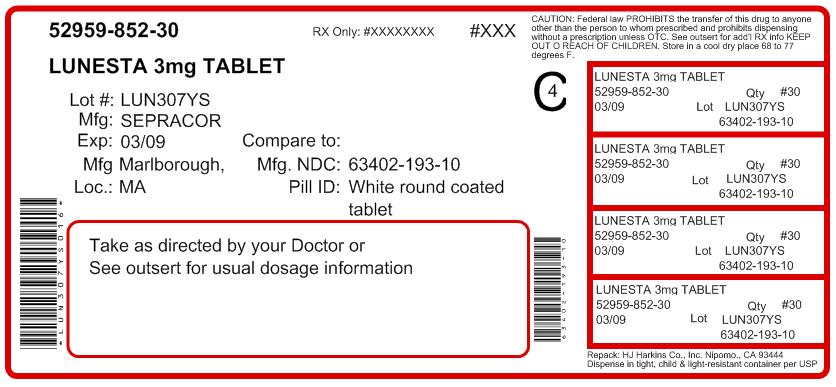
LUNESTA® (eszopiclone) TABLETS1 mg, 2 mg, 3 mg
Lunesta: Insomnia Medication Treatment (Full Prescribing Information) | HealthyPlace
Eszopiclone Vs Zopiclone — Lunesta vs. Ambien: Two Short-term Treatments for Insomnia

Insomnia: Pharmacologic Therapy - American Family Physician

Lunesta - FDA prescribing information, side effects and uses

Pharmacokinetic profiles of Z-drugs | Download Table

Insomnia: Pharmacologic Therapy - American Family Physician
Drug Criteria & Outcomes: Eszopiclone (Lunesta) Formulary Evaluation | 2005-05-01 | AHC Media: Continuing Medical Education Publishing

Generic Lunesta -
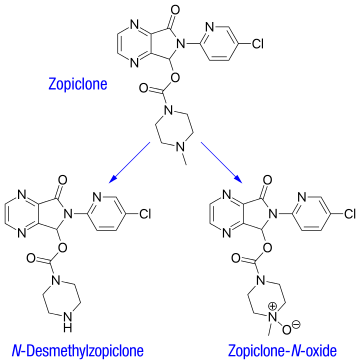
Zopiclone - Wikipedia

Drug Ads Play Up Benefits, Downsize Risks : NPR

ESZOPICLONE: Lunesta - Top 300 Pharmacy Drug Cards

FDA cuts recommended Lunesta dose in half - CNN
LUNESTA® (eszopiclone) TABLETS1 mg, 2 mg, 3 mg

Sleep Disorders in the Elderly - ppt download
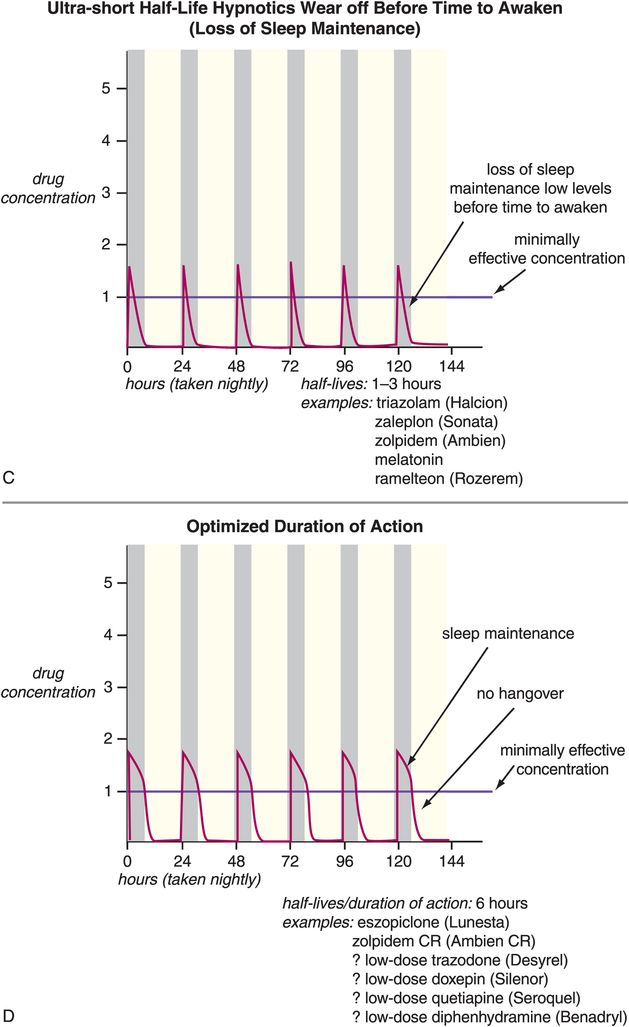
Disorders of sleep and wakefulness and their treatment | Basicmedical Key

Buy Lunesta Online - Bromazolam half life

Eszopiclone (Lunesta) - Uses, Dose, Side effects, MOA, Brands |

Ambien vs Lunesta - Difference and Comparison | Diffen

Lunesta - FDA prescribing information, side effects and uses

Assessment and Management of Sleep Disorders in the Context of Anxiety - ppt download

Helping You With Your Sweet Dreams: Lunesta (Eszopiclone) Fights Off Insomnia - Counting Sheep Sleep Research
4 Mg Lunesta‒ Insomnia Support Group|
Product Monograph Template - Standard]

Lunesta Addiction & Withdrawal

Lunesta (eszopiclone) How Long Does It Stay In Your System? | The Recovery Village Drug and Alcohol Rehab

Sepracor-Looks very promising | Stock Discussion Forums

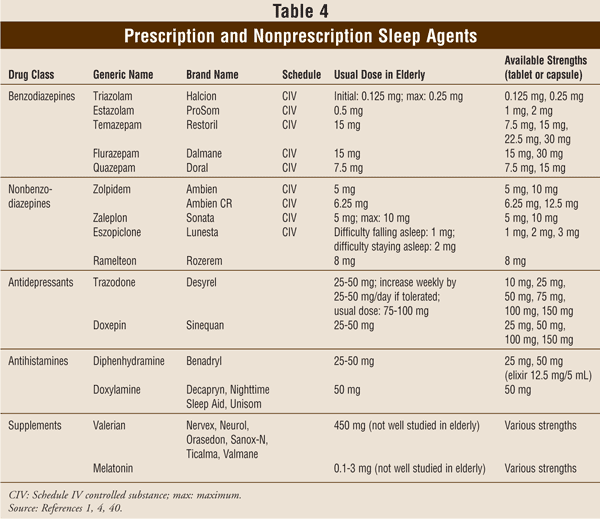
Thinking about Taking a Sleep Medication? - LHSFNA
Lunesta Prices, Coupons and Patient Assistance Programs
Posting Komentar untuk "lunesta half life"